TRANSCENTA HOLDING - A Global Fully Integrated Biotherapeutics Company
Transcenta, Biologics, Antibody, Claudin 18.2


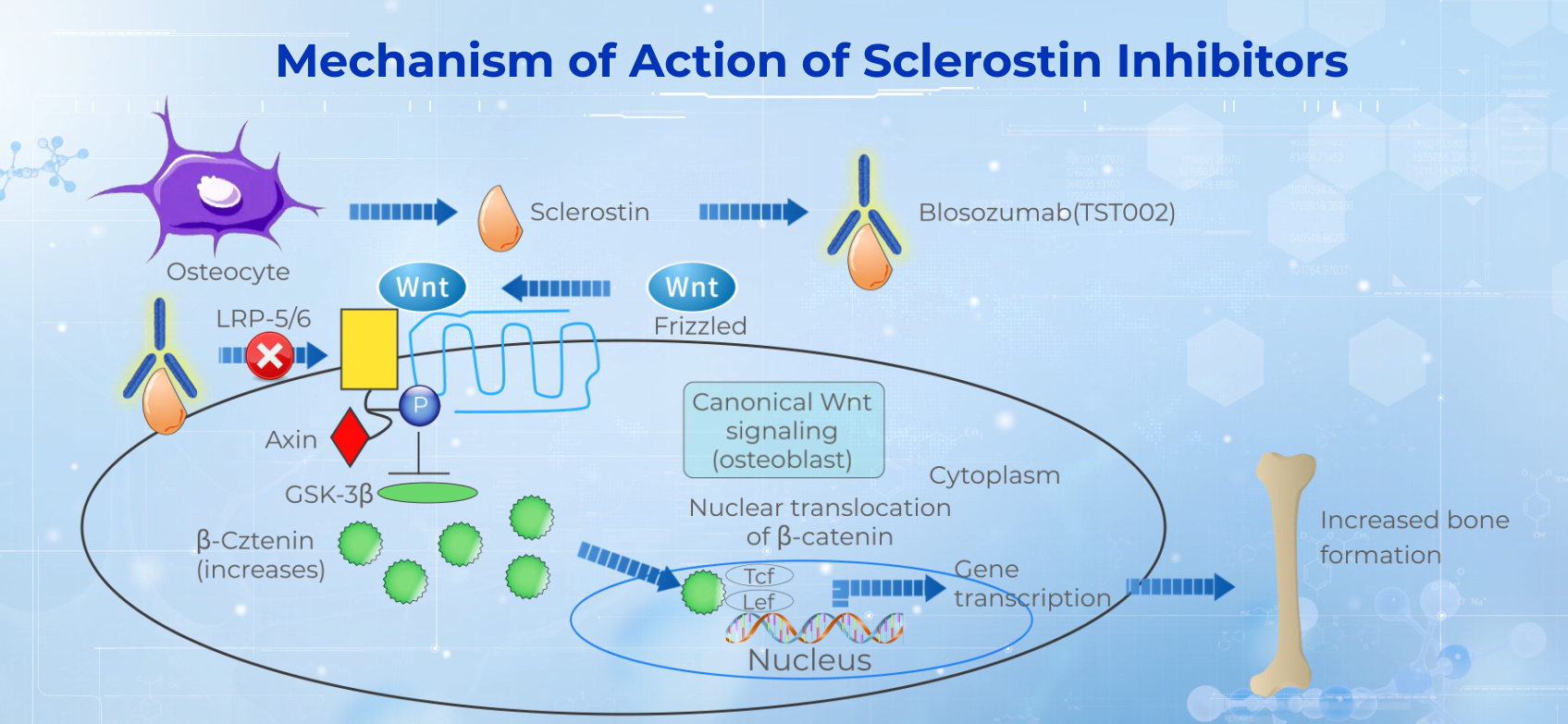
Blosozumab (TST002) is a humanized monoclonal antibody with neutralizing activity against sclerostin for which we in licensed the Greater China rights from Eli Lilly. Eli Lilly had completed Phase II trial with blosozumab in postmenopausal women in the United States and Japan. The data had shown that blosozumab can induce significant dose-dependent increases in spine, femoral neck, and total hip bone mineral density (BMD) as compared with placebo. Such studies have shown that, in the highest dose group, blosozumab treatment increased mean BMD by 17.7% at the spine, and 6.2% at the total hip from baseline after 12 months. We obtained encouraging data from 32 Chinese patients treated with a single dose of blosozumab (TST002) and followed for 85 days, including safety, bone formation and resorption markers and BMD data. After a single dose of blosozumab (TST002) up to 1,200 mg, the average increase of lumbar spine BMD at day 85 (D85) ranged from 3.52% to 6.20% and total hip BMD from 1.30% to 2.24% across dose cohorts. The safety, efficacy and PK/PD results of this study are consistent with the clinical data in the U.S. patients.
• Blosozumab (TST002) SAD study result was presented at the 2024 WCO-IOF-ESCEO Congress in April. The study result has also been presented in 2024 Chinese Society for Osteoporosis and Bone and Mineral Research Congress (CSOBMR). After a single dose of blosozumab (TST002) up to 1,200 mg, the average increase of lumbar spine BMD at day 85 (D85) ranged from 3.52% to 6.20% and total hip BMD from 1.30% to 2.24% across dose cohorts. The lumbar spine BMD increase exceeded the least significant difference level (2.77%) and was clinically meaningful.
The balance between bone resorption and bone deposition is determined by the activities of two principal cell types, namely, osteoclasts and osteoblasts. Therefore, the bone rebuilding cycle needs to start from the two aspects of inhibiting osteoclasts or promoting osteoblasts. The loss of gonadotropin with aging reduces the conversion of bone marrow stromal stem cells into adipocytes and decreases the differentiation of osteoblast precursor cells. The increased activity of osteoclasts results in osteocyte death and at the same time enhances bone resorption.
Sclerostin is a glycoprotein encoded by the SOST gene and produced in osteocytes. Sclerostin is an inhibitor of the WNT/-catenin signaling pathway, which stimulates osteoblast differentiation and bone formation. By inhibiting the activity of sclerostin, sclerostin mAbs can promote bone formation, reduce bone absorption and increase bone mineral density and bone strength, thus reverse the symptoms of osteoporosis.